New Cameras
Prince Rupert's drops
The same phenomenon is the basis of a technology that has many and profitable uses today, and is known as glass tempering. Glass as a material has a number of useful properties, and is theoretically very strong. In practice the situation is less rosy. The strength of freshly drawn glass fibres may exceed that of steel wire, but for larger glass objects (e g glass plates) it is considerably lower. Glass is particularly bad at withstanding tensile stress (stretching). The cause is the ever-present defects in the glass surface, such as microcracks. On straining, particularly high stress is concentrated to the tip of this crack and the crack may begin to propagate. Because of the particular structure of glass (as different from for instance polycrystalline metals) there is nothing to stop the propagation of such a crack, and the process ends with the fracture of the glass object. It is obvious that the larger the glass object, the greater the probability that somewhere on the surface is a defect that will prove to be fatal. This also explains the strength of glass fibres with a flame-treated surface.
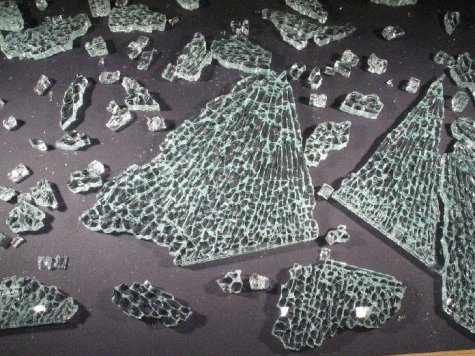
How is glass tempering carried out and why does it make glass stronger? To temper plate glass, it is heated to a temperature above 600 °C, where the glass softens, and is then rapidly cooled by blowing cold air over both surfaces of the glass plate. The first to cool and become rigid are the surfaces of the plate, while the hotter interior layers of the plate are still comparatively soft. Then the interior of the glass also starts to cool and in doing so shrinks, attempting to contract the outer surface layers. Since they however already are solid and rigid this causes strong compressive stress forces in the surface layers. It is those that make the glass stronger. For a tensile stress to become dangerous for the glass it must in the first place exceed the compressive forces in the surface layers. The glass fractures only when the total stress (the sum of the stress caused by the external force and the counteracting interior stress, with opposing sign) exceeds a critical value. Tempering glass raises its strength by about a factor 5. Tempered glass withstands hammer blows and it can tolerate quite substantial bending (Photo 4)
.In Estonia too several enterprises work with glass tempering, for instance Baltiklaas in Tartu, Klaasimeister in Harjumaa and Andrese Klaas in Tallinn. Industrially the operations connected with tempering (heating, cooling) are performed on a common conveyor line unit.
It may also be added that an effect, similar to the heat tempering described above, is achieved by so called chemical tempering. This is based on ionic exchange: on holding the glass object in a potassium salt melt, the sodium ions in the outer layers of the glass are exchanged for potassium ions. Since the Na+ and K+ ions have different volumes (K+ ions larger), this causes an internal stress in the glass. By this method only a comparatively thin (some tens of microns) surface layer is tempered. Unlike thermal tempering even complicated shapes (irregular surfaces) and very thin glass objects can be tempered. And since basically only a very thin surface layer of the glass is stressed, then such glass can also be cut and drilled. The method itself is however more time-consuming and expensive, and so is only used for smaller objects.
For more information see “Materjalimaailm” (Materials World), article “Karastatud klaas” (Tempered glass)